When
a medicine is first licensed for use in clinical practice the numbers
of patients that have been exposed is generally relatively small,
as compared to the number that will eventually receive it. Relatively
uncommon reactions may therefore not have been detected, it is primarily
for this reason that the Yellow Card Scheme
was set up. New medicines are marked in the BNF and all publicity
material with a black triangle (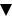 ),
and special reporting advice applies to these medicines. The are generally
described as being intensively monitored products by the CSM/MHRA. ),
and special reporting advice applies to these medicines. The are generally
described as being intensively monitored products by the CSM/MHRA.
Therefore, all reactions to any medicines with the black triangle
symbol (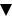 )
against it in the British National Formulary (BNF), MIMS, the ABPI
Compendium of Datasheets and Summaries of Product Characteristics
should be reported. )
against it in the British National Formulary (BNF), MIMS, the ABPI
Compendium of Datasheets and Summaries of Product Characteristics
should be reported.
Click here to see the current lists by generic
name or trade
name (PDF format  ). ).
|

